CBHA
CBHA (m-carboxycinnamic acid bis-hydroxamide) is a member of a recently synthesized family of hybrid polar compounds that have been shown to be inhibitors of HDAC and potent inducers of transformed cell growth arrest and terminal differentiation at micromolar (LD50 range, 1–4 µM) concentrations. The effective dose of CBHA that inhibited 50% clonal growth (ED50) of the endometrial cancer cell lines was calculated and ranged between 1.8 x 10-6M and 2.5 x 10-6M for CBHA.
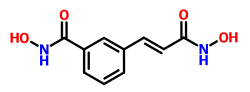
This substance induces apoptosis of human neuroblastoma in vitro and is effective in lower doses when combined with retinoids.
Synonyms: N-hydroxy-3-[(E)-3-(hydroxyamino)-3-oxoprop-1-enyl]benzamide; m-Carboxycinnamic Acid bis-Hydroxamide
Technical Data: Molecular Weight - 222.20; Molecular formula - C10H10N2O4; CAS No - 174664-65-4
Ordering Information: Product Number - EC-000.2467
Price and Availability:
Inquire
This product is also available to buy in bulk quantities.
References:
1. The histone deacetylase inhibitor, CBHA, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Coffey DC, Kutko MC, et al. Cancer Res. 2001 May 1;61(9):3591-4.
2. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. V. M. Richon, S. Emiliani, et al. Proceedings of the National Academy of Sciences of the United States of America, vol. 95, no. 6, pp. 3003–3007, 1998.
Related Products: Suberoylanilide Hydroxamic Acid; MS-275 (Entinostat); CI-994 (Tacedinaline); BML-210; M344; MGCD0103 (Mocetinostat); PXD101 (Belinostat); LBH-589 (Panobinostat); Tubastatin A; Scriptaid; NSC 3852; NCH 51; HNHA; BML-281; Salermide; Pimelic Diphenylamide; ITF2357 (Givinostat); PCI-24781; APHA Compound 8; Droxinostat; SB939.
Related service: collection of Histone Deacetylase Inhibitors.